A salt lake… great
Mars was once a water-rich world, but liquid water cannot exist on the Martian surface today due to the thin Martian atmosphere. In order for water to remain liquid under current conditions, these subsurface lakes would likely need to be extremely salty.
If confirmed by other studies, these would be the first known liquid water lakes ever found on the surface of Mars, potentially making them abodes for Martian life.
When evidence for the first lake was reported two years ago, the discovery was based on just 29 observations, taken between 2012 and 2015. This new study is compiled of the 134 observations carried out from 2012 to 2019.
“While it is not possible for water to remain stable on the surface today the new result opens the possibility that an entire system of ancient lakes might exist underground, perhaps millions or even billions of years old. They would be ideal locations to search for evidence of life on Mars, albeit very difficult to reach,” the European Space Agency reports.
Of course, the same high concentrations of salt that keep this water from freezing could also present its own challenges to life. On our own world, life thrives in the salty oceans of Earth, but few forms of life are able to withstand extreme salty conditions found in places like the Great Salt Lake.
Experiments show it takes a 50 percent solution of the salt sodium perchlorate to keep water in its liquid form at -30 Celsius. In the even colder temperatures likely to be found in the smaller lakes, another salt — calcium perchlorate — would be needed to keep water from freezing at temperatures as low as -77 C (-106 F).
“Perchlorates are ubiquitous in the Martian soil, and we thus hypothesized that a similar process may have occurred in our study region. Laboratory experiments… convincingly demonstrate that brines persist for long periods of time also when temperatures drop below the [lowest possible melting point for a substance], suggesting that, once formed, it is difficult for brines to freeze,” Pettinelli explains.
And, terrestrial life does not fare nearly as well in a calcium-rich environment as it does with sodium.
“There’s not much active life in these briny pools in Antarctica. They’re just pickled. And that might be the case [on Mars],” explains John Priscu, an environmental scientist at Montana State University.
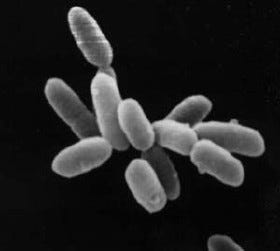
However, some extremophiles have evolved to exist in briny environments of up to 30 percent salt content. Halobacterium (“salt” or “ocean bacterium”) requires such conditions, as their proteins will not function outside the rich ionic environment provided by salt.
Questions also remain whether Mars is able to deliver enough heat to keep the lakes in a liquid state, or if the deposits of water may be closer to slush.