There’s an excellent reason why you’ve never seen someone suffering the ills of smallpox, with hard, scabbing pustules forming on their body “like peas under the skin.” It’s the same reason polio, a crippling disease, no longer exists in the U.S.
Safe and effective vaccines curbed these terrible diseases and, in the case of smallpox, globally eradicated the deadly pathogen. Vaccines continue to be critically important and trustworthy weapons for taming disease. In recent decades, dozens of vaccines approved in the U.S. have proven “remarkably safe.”
The forthcoming vaccines for COVID-19, even though they’ve been developed in record time, will meet similar standards for safety and effectiveness, according to infectious disease experts, immunologists, and bioethicists who spoke with Mashable. Yes, there’ll be well-promoted attempts to spread misinformation and sow doubt about the new COVID-19 vaccines. But if the U.S. Food and Drug Administration begins approving vaccines, which appears likely, the public should know the drugs have been rigorously tested and scrutinized for safety and efficacy.
“We’ve had a very good history of approving vaccines that have turned out to be safe,” said Katharine Van Tassel, an FDA expert and visiting professor at Case Western Reserve University’s Law-Medicine Center.
“We’re doing a good job,” added Van Tassel, who coauthored the legal guide Food and Drug Administration.
Game change. This is a great day for patients as Pfizer (where I serve on the BoD) announces final top line results from the phase 3 trial with its Covid-19 vaccine. We should now have two vaccines that could effectively end the U.S. epidemic next year if everything goes right. https://t.co/ieQwYywD97
— Scott Gottlieb, MD (@ScottGottliebMD) November 18, 2020
Why get a COVID-19 vaccine in the first place?
The coronavirus pandemic will not end, for years, without a vaccine. “At this point in time, given how we’re struggling with the pandemic, the vaccine is our best hope to get back to some semblance of normal,” said Johan Bester, a formerly practicing emergency physician and now a bioethicist at the University of Nevada, Las Vegas.
The devastating flu pandemic in 1918 and 1919 eventually went away — after killing at least 50 million people globally. But this influenza virus was much different than the coronavirus: Flu viruses mutate relatively quickly, so this horrid 1918 strain likely evolved into a less lethal strain, and widespread infections during the extreme outbreaks provided broad natural immunity, too. Yet we can’t expect this coronavirus to fade away like the constantly-changing flu. Our other public health weapons — namely social distancing and masking — have unquestionably slowed the spread of disease, but as winter sets in and people spend more time indoors (and unwisely gather for the holidays), these tools aren’t enough: A potent third surge has swept the U.S.
“We need another tool. We need something else,” explained Mark Cameron, an immunologist at Case Western Reserve University who previously helped contain the outbreak of another deadly coronavirus, SARS, in 2003. “This virus is getting away from us.”
This tool is a vaccine or vaccines, Cameron emphasized. To successfully curb this coronavirus, we must develop a “herd immunity” (perhaps around 70 percent of the populace) against the spread of disease, making it unlikely for the virus to move through the population. Without vaccines, the other option for creating mass immunity is simply letting everyone become infected and allowing our bodies to naturally produce immunity. But that will inevitably result in mass death, because the coronavirus is much deadlier (“possibly 10 times or more”) than most flu strains. That’s simply an unacceptable amount of suffering. “People have to understand that we’re not getting to herd immunity by natural infection alone,” said Cameron.
Generally speaking, getting vaccinated isn’t just about your own protection. Not everyone can be vaccinated for various diseases, like people with compromised immune systems or infants. Protecting the vulnerable in our society, then, depends on widespread immunity. “They depend on herd immunity to be safe,” said Bester.
Why trust a quickly-approved vaccine?
Two companies that produced remarkably promising vaccine candidates, a Pfizer and BioNTech collaboration and the biotechnology company Moderna, have already asked the FDA for an Emergency Use Authorization, or EUA, to make the vaccines available much faster than they otherwise would be during non-urgent times. If approved, however, this doesn’t mean the drugs have skipped deep scrutiny and testing.
“All vaccines have to go through rigorous testing,” said Lisa Lee, a research professor in the Department of Population Health Sciences at Virginia Tech. Lee was the executive director of the Presidential Bioethics Commission during the Obama administration.
The term “Emergency Use Authorization” might be misleading jargon for those unfamiliar with the complexities of the FDA. But it doesn’t imply that a brand new vaccine will be forced through the vetting process. Rather, these drugs are prioritized by the agency to address a public health crisis when there are no other alternatives. “‘Emergency use’ gives people the impression that the process was shortchanged, and that’s not true,” said Lee. “It puts these kinds of products [like new vaccines] in the front of the line. It doesn’t mean any corners are cut.”
The vaccines must still pass through assessments from four bodies of scientists, explained the FDA expert Van Tassel:
1. Data Safety Monitoring Board (DSMB)
During COVID-19 clinical trials (the time when vaccines and placebos are given to people) a unique DSMB board evaluates the safety and effectiveness of each vaccine. “It’s a completely independent board,” said Van Tassel, noting it’s comprised of experts like biostatisticians, bioethicists, and immunologists. The panel, whose members are often kept confidential to avoid any political or other interference, review the trials and recommend continuing, or if necessary, halting the tests (if the drug is potentially unsafe or causing illness). In the case of COVID-19 vaccines funded with taxpayer dollars, like Moderna’s, the National Institute of Allergy and Infectious Diseases, headed by Dr. Anthony Fauci, picks this board.
(If the trials aren’t paid for with taxpayer money, like Pfizer’s vaccine, that company will set-up its own DSMB review board, explained Van Tassel. Most clinical trials in the U.S., which don’t have prodigious pandemic-inspired funding, are run by the industry, which could potentially lead to biased results or conflicting interests. Pfizer maintains its COVID-19 DSMB is “a committee of independent scientists.” The company has incentives to produce trustworthy, reliable results if they ultimately want the FDA to authorize the vaccine.)
2. FDA
When the Data Safety Monitoring Board is satisfied with the results of the trials (for example, no severe reactions to the drugs), it sends all that information to the FDA. Then, a drug company (like Moderna) can ask for an Emergency Use Authorization. The FDA’s toxicologists, chemical manufacturing experts, virologists, and beyond scrutinize how well the drug worked, or didn’t. “In all of this they’re looking at safety data,” said Van Tassel.
Pfizer and Moderna completed their clinical trials. The FDA is now evaluating their requests for Emergency Use Authorization (as of Dec. 2).
3. Vaccines and Related Biological Products Advisory Committee (VRBPAC)
A separate, independent committee, the Vaccines and Related Biological Products Advisory Committee, is also convened by the FDA to evaluate the safety and effectiveness of the vaccine. “These people can’t have any conflict of interest,” said Van Tassel. They are 15 experts in science and public health, and contain no political appointees. Ultimately, they’ll recommend to the FDA if a vaccine should be given Emergency Use Authorization. If so, the FDA commissioner will likely heed their advice, and approve the vaccine.
4. The CDC
But that’s not all. Once the drug is approved, the Centers for Disease Control and Prevention (CDC) has a board, called the Advisory Committee on Immunization Practices, to provide guidance on how and when the vaccine should be used. For example, the CDC recommended health care workers and elderly people in long-term care facilities receive vaccinations first, as supplies will be limited.
The public health experts assessing the safety of a COVID-19 vaccine have a profound incentive to ensure the drugs are safe — if they have an authentic interest in advancing public health, anyhow. “All these scientists are very aware that if they make a mistake, then people will not want to take any future vaccinations,” said Van Tassel. “The future of vaccination is in their hands.”
“The future of vaccination is in their hands.”
In addition to this official vaccine scrutiny comes an exceptional interest from hundreds, if not thousands, of outside experts, particularly in academia and other research institutions. If the FDA approves a vaccine, all the clinical data becomes public and open to analysis. If something is awry, it will be caught. Any significant problem will likely go viral on Twitter, too.
“It would be flagged very quickly,” said UNLV’s Bester. “There is very little scope to hide something. They’re working under a bright spotlight.”
What happens after the COVID-19 vaccines are approved?
Once a vaccine is out in the wild, the scrutiny doesn’t end. Public health agencies keep watching to ensure the drugs are safe. “We do a great deal of monitoring of vaccines,” said Virginia Tech’s Lee.
There are three important ways any potential problems with vaccines, like an adverse reaction, can be reported to public health agencies, she explained.
-
Vaccine Adverse Event Reporting System (VAERS): This allows anyone to report potential safety problems with a vaccine to the CDC and FDA.
-
Doctors are legally required to report any dramatic or severe events from vaccinations.
-
V-SAFE: The CDC is launching a new app to check in with people after they’ve received a forthcoming COVID-19 vaccine. The system “will provide telephone follow up to anyone who reports medically significant (important) adverse events.”
But isn’t this a new type of vaccine?
Two companies (Pfizer and Moderna) that have announced promising results from their clinical trials are using a new generation of vaccine, called mRNA vaccines. They’ve never been approved in the U.S. But that doesn’t mean the new vaccines are unsafe. Rather, they’ve proven remarkably safe and effective so far. Moderna’s large-scale 30,000-person trial resulted in 94.5 percent efficacy, meaning a 94.5 percent reduction in disease compared to the unvaccinated group. What’s more, no one infected with the coronavirus who received the Moderna vaccine experienced severe symptoms of COVID-19 (meaning the vaccine protected against the worst effects of an infection, too). Meanwhile, on Dec. 2 the UK became the first Western nation to approve Pfizer’s mRNA vaccine, which showed a 95 percent efficacy and only one case with severe symptoms in the vaccinated group.
Traditionally, vaccines involve injecting a weakened or inactivated virus into our bodies, which allows our immune system to “see” the virus and prepare defenses against this pathogen. These classic vaccines (like the measles vaccine) cause a relatively harmless infection, triggering an immune response so you’re ready for the real virus, when it comes.
An mRNA vaccine triggers an immune response too, but in a different way. RNA is a strand of genetic material, or code, that exists in all of our cells, and many viruses too. It’s hugely important: RNA gives instructions for making our proteins (the complex molecules critical for running our organs and tissues). For a COVID-19 mRNA vaccine, researchers have done something clever: They’ve used a specific section of the coronavirus RNA to make a protein in our body that ultimately triggers an immune response. It’s literally sending instructions to our cells for how to fend off a dangerous pathogen.
“It’s biological code,” said John Cooke, the medical director of the RNA Therapeutics Program in the Houston Methodist DeBakey Heart and Vascular Center. “It’s like writing software.”
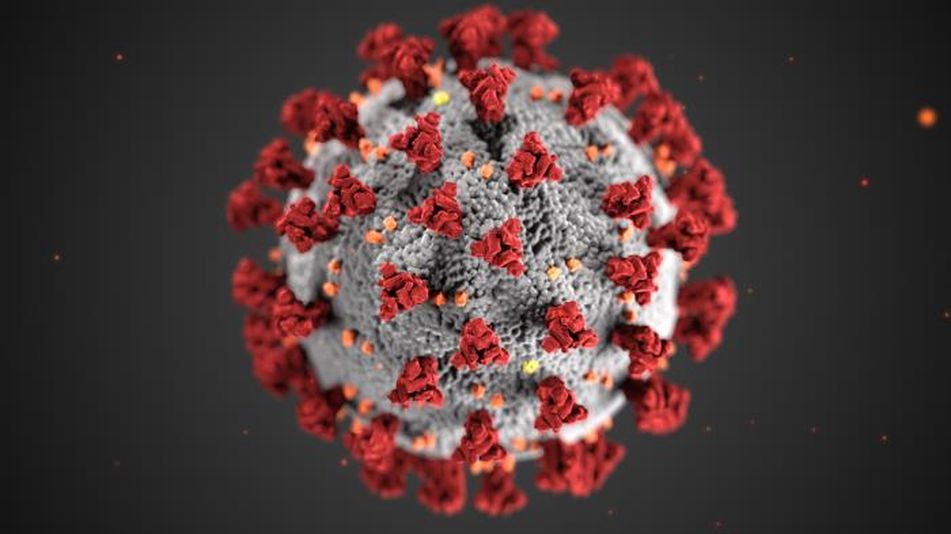
Specifically, to create the vaccine researchers code for (and then produce in a lab) the exact strip of RNA needed to make the coronavirus’ infamous spike protein, found on the outside of the virus. The virus uses this spike to bind with and then enter our cells, like a key opening up a locked door.

A rendering of the spikes on the coronavirus.
Image: cdc / Alissa Eckert, MSMI / Dan Higgins, MAMS
Once in our bodies, the mRNA vaccine promptly triggers immune action. The cells in our upper arm, where the vaccine is given, take up and proceed to copy the code and make bounties of this spike protein. The cells then release the spike proteins, ultimately allowing our immune system to recognize and create defenses against the spikey coronavirus — if and when it arrives. (Our immune systems, for example, can then make antibody proteins that block the coronavirus spikes from latching onto our cells and eventually causing disease symptoms.)
Using mRNA vaccines isn’t like sending some unnatural invader into our bodies. Quite the opposite. “RNA is something our cells make all the time,” said Cooke. “We wouldn’t survive without it. Your body knows how to deal with RNA.”
The mRNA vaccines were rapidly developed and advanced to clinical trials in record time, noted Cameron, of Case Western Reserve University. That’s because the mRNA process is streamlined: Researchers only had to identify, and then make, one piece of code, or “genetic software.” The vaccines worked well in animals, and then proved safe and effective in clinical trials. Now, the vaccines are already under FDA scrutiny (previously, the mumps vaccine was the fastest vaccine ever developed, and that took four years).
“It’s so accelerated,” marveled Cooke, noting Moderna started trials just 42 days after sequencing the virus’ genetic code. “That is unheard of.”
Another exciting Monday morning for COVID-19 vaccines! Moderna is reporting 94.5% efficacy for their mRNA vaccine.
Read on for a biostatistician’s breakdown of the interim results. 10 tweets on severe disease, subgroup analysis, and more.
Press release: https://t.co/8lQR5eSW1r pic.twitter.com/56HGZhHXBk
— Natalie E. Dean, PhD (@nataliexdean) November 16, 2020
For all the drudgery of 2020, at least we live in an age of ever-advancing vaccines. It behooves us to take full advantage of technology that averts misery, sickness, and death.
Measles, polio, Hepatitis B were once rampant in the U.S. “These diseases circulated freely among the population,” said UNLV’s Bester. “What stopped their spread was the introduction of effective vaccines.”
The same will be true for the latest coronavirus outbreak, the most recent — but far from last — pandemic. You’ll be far more resilient against the new coronavirus entering your cells and rapidly spreading. After all, the coronavirus is a parasite. It needs you, a host, to survive and multiply. Don’t let it.
“We have confidence in the results so far,” said Bester. “I would have no hesitation getting a vaccine.”