On Thursday, the Food and Drug Administration made a decision that’s likely to have a major impact on the landscape of Alzheimer’s disease research. The agency issued a full-throated traditional approval of the drug Leqembi, developed by the companies Eisai and Biogen. The drug is the first of its class to receive such approval and is intended to slow down the progression of the neurodegenerative disease.
Leqembi is one of several antibody-based drugs that target amyloid beta, a protein that plays an important role in Alzheimer’s. In those with the disease, a misfolded form of amyloid builds up in the brain over time, causing the development of hardy clumps called plaques. These plaques, along with the accumulation of another misfolded protein called tau, are thought to help gradually destroy the brain. By breaking down or preventing the formation of plaques, it’s hoped that these drugs can stop or slow people’s cognitive decline.
Advertisement
In January 2023, the FDA issued an accelerated approval of Leqembi. This type of approval allows companies to only present indirect evidence that their drug will be clinically meaningful to patients—in this instance, the reduction of amyloid plaque. But companies are still required to collect data and eventually confirm a drug’s clinical benefits in order to receive traditional approval. And it appears that Leqembi has now met that benchmark.
“Today’s action is the first verification that a drug targeting the underlying disease process of Alzheimer’s disease has shown clinical benefit in this devastating disease,” said Teresa Buracchio, acting director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, in a statement released Thursday.
Advertisement
Advertisement
In the pivotal 18-month-long clinical trial that secured Leqembi’s approval, the drug was found to slow the progression of cognitive decline by 27% in patients compared to those on placebo. Patients also performed better on tests of their daily functioning and had lower levels of amyloid in their brains.
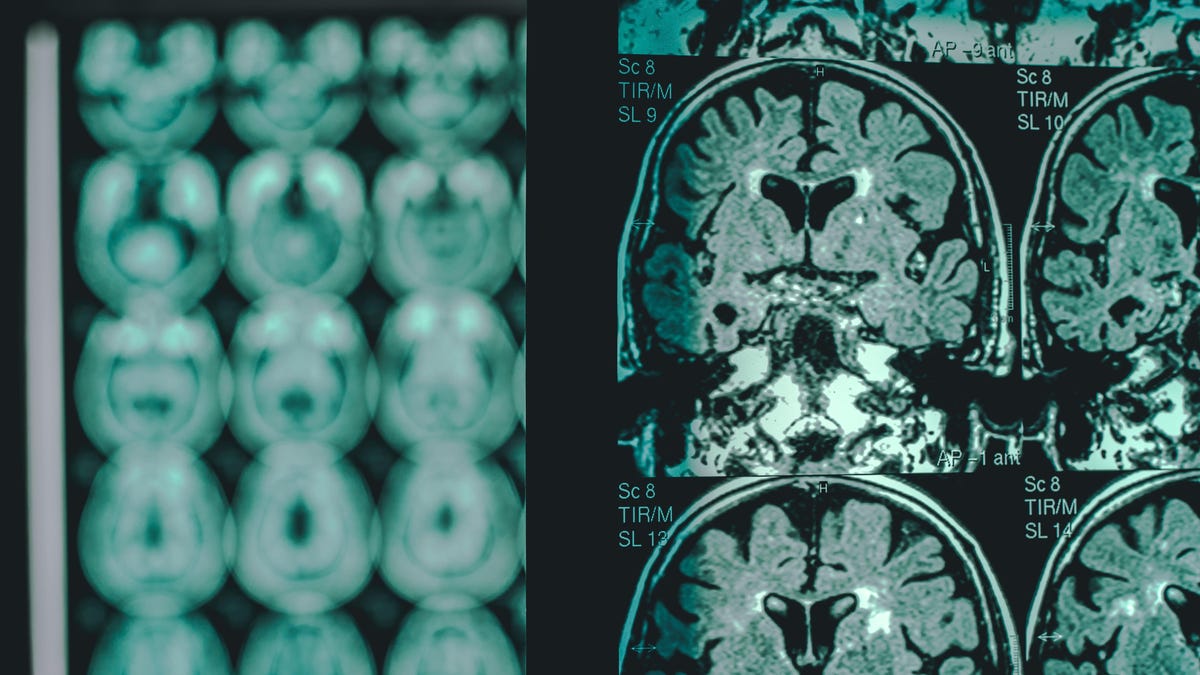
Anti-amyloid drugs aren’t without their side effects, however. One of the most common complications is known as amyloid-related imaging abnormalities (ARIA), which can be diagnosed via MRI. ARIAs tend to be caused by temporary swelling in the brain, but sometimes, they can be a sign of life-threatening bleeding. Most cases of ARIA resolve without problem, with many patients experiencing no symptoms, but there have been several deaths linked to ARIAs and these drugs.
The risk of ARIA and severe ARIA seems to be higher in those carrying a particular Alzheimer-related mutation called ApoE ε4. The use of blood thinners might also be another risk factor for severe brain bleeding in these patients. As a result, the drug’s labeling will call for doctors to test patients’ ApoE ε4 status before prescribing it and will recommend added caution for considering its use in those taking blood thinners.
The traditional approval of Leqembi will sidestep a controversy surrounding these anti-amyloid drugs. In June 2021, the FDA issued an accelerated approval to the drug Aduhelm, also developed by Biogen and Eisai. The data supporting Aduhelm’s approval was decidedly weak and many outside experts (including a majority of those appointed by the FDA to advise the agency) protested the decision. Eventually, Medicare ruled that it would not routinely cover Aduhelm and similar drugs given accelerated approval until clear evidence of its benefits was collected. The FDA was later harshly criticized by lawmakers for its “irregular” approval of the drug, and the drug’s makers have delayed plans to seek approval elsewhere.
Advertisement
Though Aduhelm may never receive full FDA approval and routine insurance coverage, the new Medicare policy will now no longer apply to Leqembi. The drug’s current list price ($26,000 a year) is also half as much as the initial price of Aduhelm, another factor that fueled widespread criticism of the latter. That said, some researchers have continued to argue that Leqembi’s clinical benefits are likely too modest for patients and doctors to be very excited about for the time being. But the drug class does appear to be improving. Earlier this May, Eli Lilly’s donanemab provided the best results of its kind seen yet, reducing people’s rate of cognitive decline by 35% compared to a placebo in a large-scale trial.
Services Marketplace – Listings, Bookings & Reviews