Anyone who has ever experienced phantom ringing in their ears knows that it is a nuisance to say the least. Those who have tinnitus – hearing continuous ringing, buzzing, humming or even roaring sounds – often experience anxiety and depression as a result.
The condition affects approximately 15% of the global adult population. However, treatment has remained elusive, with those afflicted left to find their own ad hoc mitigation solutions.
Neuromod, a medtech startup from Ireland, is looking to change that. The company has just received €30 million in funding to further commercialise its tinnitus treatment device, Lenire.
A different kind of electrotherapy
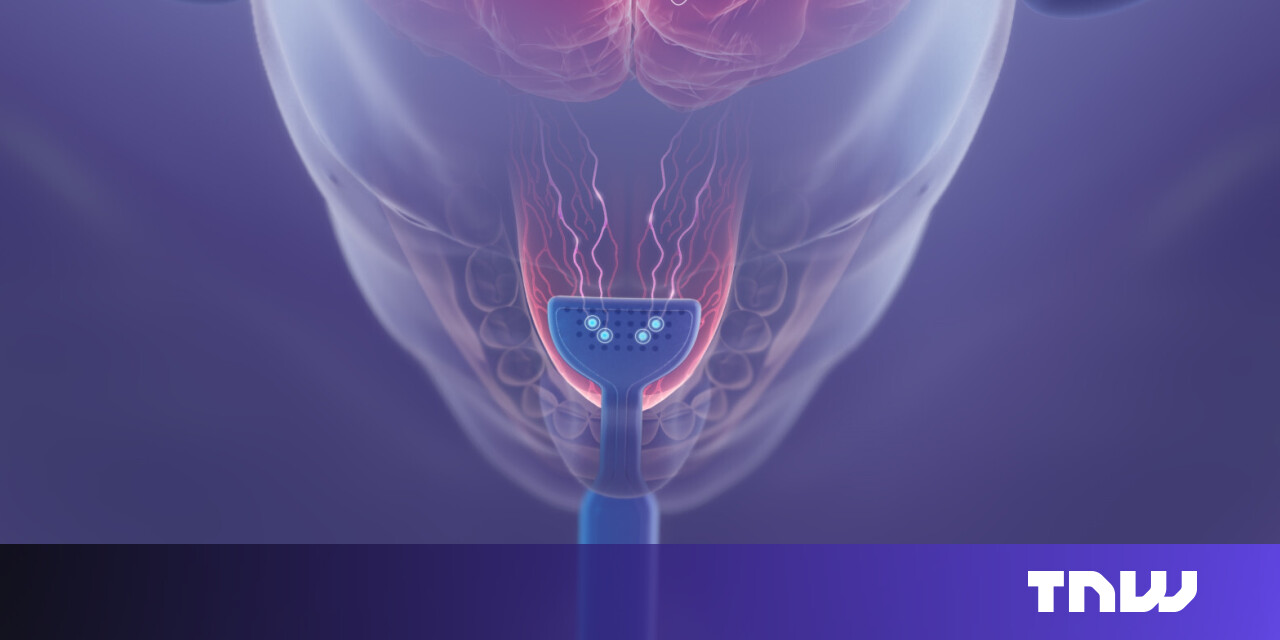
With its patented bimodal neuromodulation technology, Lenire works by sending mild electrical signals to the tongue, while patients listen to auditory stimulation through headphones.
Thus far, over 700 patients have participated in clinical trials with the device, which consists of three parts. A handheld, lightweight controller allows the user to control timing, intensity and synchronisation of the stimuli, while Neuromod’s proprietary Tonguetip module sits in the user’s mouth, administering electrical pulses to the top of the tongue. Simultaneously, Bluetooth headphones deliver customised sound stimuli to the auditory nerve.
<iframe srcdoc="*{padding:0;margin:0;overflow:hidden}html,body{background:#000;height:100%}img{position:absolute;top:0;left:0;width:100%;height:100%;object-fit:cover;transition:opacity .1s cubic-bezier(0.4,0,1,1)}a:hover img+img{opacity:1!important}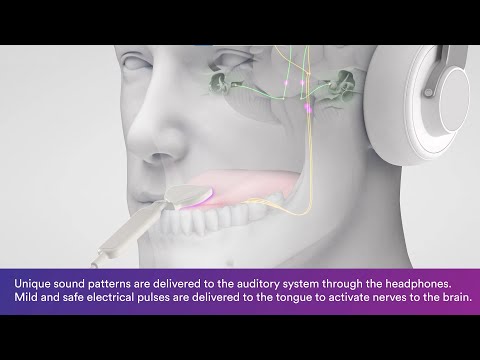
 ” height=”240″ width=”320″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture” allowfullscreen frameborder=”0″>[embedded content]
” height=”240″ width=”320″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture” allowfullscreen frameborder=”0″>[embedded content]
Large-scale clinical studies of Lenire, featured in the October 2020 edition of Science Translational Medicine and the June 2022 edition of Nature, found that between 70% and 86% of participants reported a reduction in symptoms. Furthermore, the decrease in discomfort persisted over a post-therapeutic study phase which lasted up to 12 months.
Taking Neuromod across the Atlantic
As with most medtech, due to regulatory procedures, the company’s trajectory from inception to trials to market is somewhat longer than for startups in other sectors.
Neuromod Devices was founded in 2010, and the funding raised this week brings the total capital raised to over €55 million. The latest round consists of €15 million in equity investment and €15 million in venture debt, with the latter provided by the European Investment Bank.
The equity investment is led by Panakés Partners, a venture capital firm based in Milan, with the expressed goal of “providing a better life to people all around the world.” Panakés Partners’ managing director Alessio Beverina will join Neuromod’s board.
Existing investor Fountain Healthcare Partners also participated in the expansion of the Series B funding.
With the previous round of Series B funding, which took place in 2020, Neuromod used the funds to expand its presence across Europe. This time, while still looking to increase accessibility to the device in new European markets including the Netherlands, Sweden, and Italy, the funds will also support the launch of Lenire in the US.
The company has already established a wholly owned subsidiary, Neuromod USA Inc, and gained De Novo approval from the FDA. Initial patient treatment in the US will begin this month.
Tinnitus treatment is one of the largest unmet clinical needs in the world. For some of the millions of people suffering from phantom sounds around the clock, perhaps Neruomod’s Lenire could provide relief from the constant uninvited companion in their ears.
Services Marketplace – Listings, Bookings & Reviews