
Withings is the unsung hero of the health-focused smartwatch world, with its gorgeous, hybrid, and truly watch-like Withings ScanWatch often flying under the radar of those beguiled by the digital Apple Watch Series 8 or Samsung Galaxy Watch 6. Now, Withings has introduced the next-generation ScanWatch 2, along with a tempting, reasonably priced new model called the ScanWatch Light.
Both look and operate very differently from those all-digital rivals, and Digital Trends spoke to Withings’ head of consumer products, Matthieu Menanteau, ahead of the launch to learn more.
Meet the ScanWatch 2 and ScanWatch Light

First, we should go through some of the core specifications and differences between the new models, which, again, are the ScanWatch 2 and the ScanWatch Light. We’ll start off by discussing the ScanWatch 2, which has the most updates and new technology inside.
It comes in 38mm and 42mm case sizes, each with physical hands on the dial, along with a 0.63-inch grayscale OLED screen as a complication. The dial has sapphire crystal over the top, while the case and crown are made from stainless steel. On the back is an upgraded 16-channel PPG sensor and a new TempTech24/7 temperature sensor, plus the smartwatch has an upgraded accelerometer, along with an altimeter.

There’s a choice of colors and designs. The 42mm version comes in a silver/black combination, with a silver/white version to follow, and has integrated lugs and a more noticeable, flatter bezel. The 38mm version has a bar-style lug design and comes in silver/white, silver/black, and rose gold/sand, with a rose gold/blue model to come in the future. The strap is made from fluoroelastomer and has a stainless steel buckle.
The ScanWatch Light only comes in a 37mm case, which is also made from stainless steel, but it’s Gorilla Glass over the dial. It has the same OLED screen complication. The case back has an accelerometer and a PPG sensor, but not the upgraded version or the TempTech24/7 sensor. The dial is missing the second hand complication, and the design is simpler overall. It comes in silver/black, silver/white, and rose gold/sand colors. Both new ScanWatch smartwatches have a battery expected to last for 30 days between charges and a two-hour recharge time using the included charging dock. Plus, they are both water resistant to 50 meters and compatible with iOS and Android.
A new way to measure body temperature

I spoke to Menanteau a few weeks before the ScanWatch 2’s launch, and he gave me a lot of insight into what’s new.
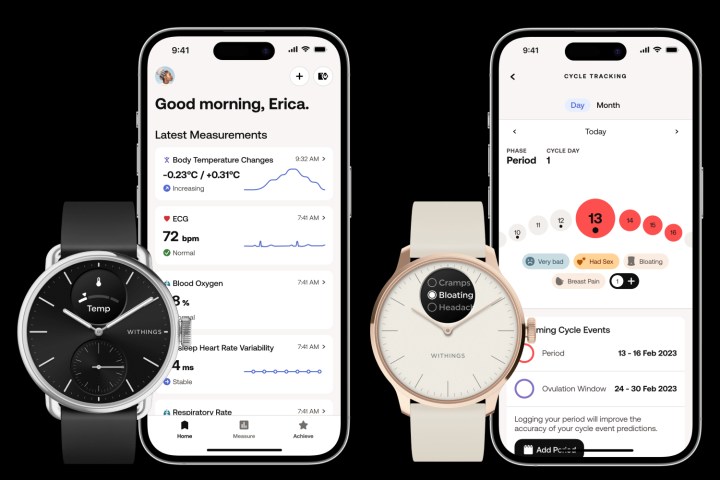
“The breakthrough innovation is the body temperature reading, using what we call the TempTech24/7 sensor, a technology that combines four hardware sensors, the first one being a classic temperature sensor that measures skin and ambient temperature. The second one is a heat flux sensor exclusive to Withings, which measures the energy transit between the watch and the body. The watch will also measure your heart rate and motion level. These four parameters will enable 24/7 body temperature measurement, so you’ll be able to monitor changes during the day and night.”
The TempTech24/7 sensor has been developed by Withings and a Swiss technology company named GreenTEG. Menanteau continued to explain why it’s a big deal:
“So that’s the breakthrough. Body temperature is one of the key vitals, but it’s a world first that it’s on the wrist, and it’s not skin temperature, and it’s 24/7. We are proud to bring that to our customers,” he told Digital Trends. “We’ve also been adapting the workout algorithm, and we’ve been building the everyday living algorithm to give a complete and comprehensive picture of your body temperature across the night.”
Other important health upgrades
The TempTech24/7 sensor is crucial to the Withings ScanWatch 2, but what about the rest of the sensors and technology?
“We’ve completely redesigned the optical sensor,” Menanteau continued., “Plus, there is a new accelerometer to help trigger the activity recognition and other algorithms. We call it a high dynamic range accelerometer, and it’s much more precise. With it, we’ve been able to increase the number of activities being automatically detected and the precision of detection. And last but not least, there is a brand new screen on the watch.”
Behind the new and updated sensors is a new piece of software, ready to interpret the signals on your wrist.
“HealthSense has a new algorithm to go along with the temperature capabilities and the new PPG [heart rate sensor] LED capabilities, which give the ability to get a heart rate variability (HRV) measurement and a respiratory rate measurement. [We have also] enhanced the electrocardiogram (ECG) capabilities with new outputs, so it’s more precise and we have more diagnosis capabilities.”
Withings is also introducing a new cycle tracking system with the ScanWatch 2.
“We’re really proud to bring menstrual cycle tracking capabilities with a manual log feature that’s only two clicks away, where you will be able to log whether you have your period and your symptoms, Menanteau said. “It will sync with the app, where you’ll be able to manage and follow what happens to your body.”
What about the ScanWatch Light?

If the ScanWatch 2 is beyond your needs, you may be considering the Withings ScanWatch Light, but just how different is the cheaper version? Menanteau provided more insight:
“The ScanWatch Light is still crafted from premium materials and has premium sensors. We’ve removed the advanced temperature and blood oxygen monitoring for those who currently don’t want to monitor those biomarkers. We kept the breathing disturbances because we want to focus on the activity and the sleep tracking, and we are keeping the cycle-tracking features, along with the continuous heart rate tracking,” he confirmed.
FDA approval, competitive prices

When Withings released the original ScanWatch, it took a while for it to gain Food and Drug Administration (FDA) approval in the U.S., so what’s the situation with the ScanWatch 2?
“The ScanWatch 2 is FDA-approved,” Menanteau confirmed, “for atrial fibrillation detection through ECG recording.”
The situation around FDA approval is complicated. The original ScanWatch was also FDA-approved for blood oxygen measurements, but the ScanWatch 2 hasn’t been at this time, only to ensure Withings could release the smartwatch in a timely manner. It does intend to get approval in the future. Menantaeu said the ScanWatch 2’s performance is better than the ScanWatch’s, so he’s not concerned about it gaining approval. The heart rate sensor is not subject to mandatory FDA approval, unlike the ECG component.
“All key metrics have been clinically tested and some of them are clinically validated, meaning they are FDA or CE [for the European Economic Area] regulated and approved,” Menanteau said, clarifying any concerns over accuracy and approval.
The ScanWatch 2, in either case size, costs $350 or 320 British pounds, while the ScanWatch Light costs $250 or 230 pounds. Both will be released in October.
Editors’ Recommendations
Services Marketplace – Listings, Bookings & Reviews
