Crispr’s potential for curing inherited disease has made headlines, including at WIRED, for years. ( Here, here, here, and here.) Finally, at least for one family, the gene-editing technology is turning out to deliver more hope than hype. A year after 34-year-old Victoria Gray received an infusion of billions of Crispr’d cells, NPR reported last week that those cells were still alive and alleviating the complications of her sickle cell disease. Researchers say it’s still too soon to call it a cure. But as the first person with a genetic disorder to be successfully treated with Crispr in the US, it’s a huge milestone. And with dozens more clinical trials currently in progress, Crispr is just getting started.
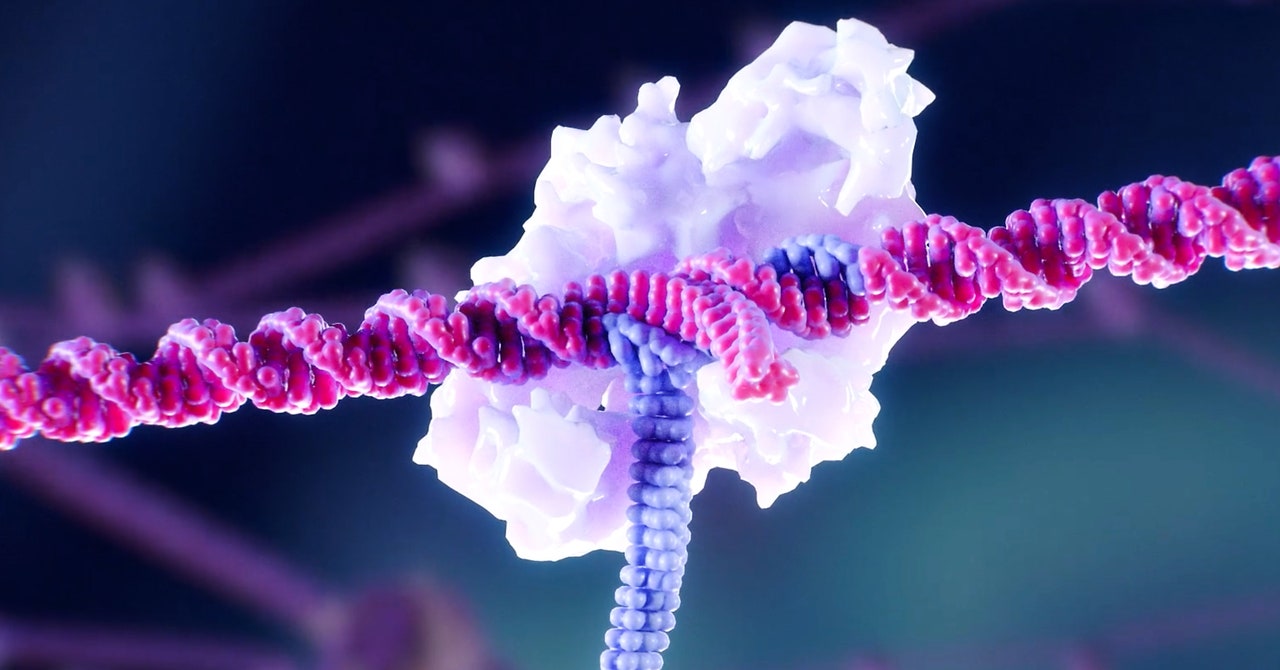
Yet for all its DNA-snipping precision, Crispr is best at breaking DNA. In Gray’s case, the gene editor built by Crispr Therapeutics intentionally crippled a regulatory gene in her bone marrow cells, boosting production of a dormant, fetal form of hemoglobin, and overcoming a mutation that leads to poor production of the adult form of the oxygen-carrying molecule. It’s a clever way around Crispr’s limitations. But it won’t work for a lot of other inherited conditions. If you want to replace a faulty gene with a healthy one, you need a different tool. And if you need to insert a lot of DNA, well, you’re kind of out of luck.
Not anymore, says Geoffrey von Maltzahn, the CEO of a new startup called Tessera Therapeutics. The company, founded in 2018 by Boston-based biotech investing powerhouse Flagship Pioneering, where von Maltzahn is a general partner, emerged from stealth on Tuesday with $50 million in initial financing. Tessera has spent the past two years developing a new class of molecular manipulators capable of doing lots of things Crispr can do—and some that it can’t, including precisely plugging in long stretches of DNA. It’s not gene editing, says von Maltzahn. It’s “gene writing.”
“Simplistically, we think of it as a new category,” says von Maltzahn. “Gene writing is able to make either perfect deletions or simple base pair changes, but its wheelhouse is in the full spectrum, and in particular the ability to make large alterations to the genome.”
To get beyond simplistics, to understand how gene writing works, you have to take a deep dive into the history of an ancient, invisible battle that’s been raging for billions of years.
For nearly as long as there have been bacteria, there have been viruses trying to attack them. These viruses, called phages, are like strings of malicious computer code trying to hack into a bacterial genome to trick it into making more phages. Every day, phages invade and blast apart huge quantities of the world’s bacteria (up to 40 percent of the bacterial population in the oceans alone). To avoid the unrelenting slaughter, bacteria have had to constantly evolve defense systems. Crispr is one of them. It’s a way for bacteria to steal a bit of a phage’s code—its DNA or RNA—and store it in a memory bank, like a primordial immune system. It’s the longest-running arms race in the history of Earth, says Joe Peters, a microbiologist at Cornell University: “That level of evolutionary pressure has driven an incredible amount of novelty in molecular mechanisms for manipulating DNA and RNA.”
But bacteria haven’t just had to contend with foreign viral invaders. Their genomes are also under perpetual assault from within. Through the millennia, as bacteria have been swapping bits of DNA with each other, trying to stay ahead of the next wave of phage attacks, some of those genes evolved the ability to move around and even replicate independently of the rest of their original genome. These so-called “mobile genetic elements,” or MGEs, carry self-contained code for the machinery to either cut and paste or copy and paste themselves into a new locality, either within their host or into nearby bacteria.